Deterioration mechanism of LiNi0.8Co0.15Al0.05O2/graphite–SiOx power batteries under high temperature and discharge cycling conditions†
Abstract
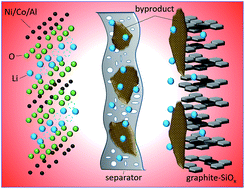
LiNi0.8Co0.15Al0.05O2 and graphite–SiOx composites have been considered as potential cathode and anode materials for next-generation batteries due to their high specific capacity. It is significant to illustrate the degradation mechanism of NCA/graphite–SiOx power batteries under various conditions for their wide application. In this study, 10 A h NCA/graphite–SiOx power batteries were prepared and their deterioration mechanism at different temperatures (25 °C, 45 °C, and 65 °C) and discharge rates (1C and 3C) was systematically investigated. The results show that the batteries experienced 15.02% and 52.17% capacity loss after 400 cycles at 25 °C and 45 °C at 1C, respectively, and 21.94% after 400 cycles at a discharge rate of 3C. The capacity loss is as high as 79.93% after only 150 cycles at 65 °C and 1C. The long-term cycling behavior of the batteries is strongly affected by temperature, whereas it is negligibly affected by the discharge rate. The reversible lithium loss from the NCA cathode and structure decay of graphite–SiOx are responsible for the capacity loss of the battery. Many lithium alkyl carbonate (Li2CO3 and ROCO2Li), fluorophosphate (LixPOyFz and LixPFy), LiF, and oxygenated species are observed on the surface of graphite–SiOx due to the decomposition of the electrolyte at high temperatures. These species are deposited on the separator and thus block the pores and hinder ion transport; this results in a great increase of battery resistance and sudden loss of battery capacity.


 Please wait while we load your content...
Please wait while we load your content...