Post-synthetic functionalization of tryptophan protected peptide sequences through indole (C-2) photocatalytic alkylation†
Abstract
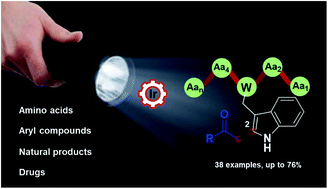
We report a selective, mild, and efficient C–H functionalization of tryptophan and tryptophan-containing peptides with activated α-bromo-carbonyl compounds under visible-light irradiation. The protocol efficiency is outlined by the wide substrate scope and excellent tolerance of sensitive functional groups present in the amino acid side chains. The method can be successfully extended to access pharmaco-peptide conjugate scaffolds.
- This article is part of the themed collection: Chemical Communications HOT articles


 Please wait while we load your content...
Please wait while we load your content...