Optical properties of Mn2+ doped cesium lead halide perovskite nanocrystals via a cation–anion co-substitution exchange reaction†
Abstract
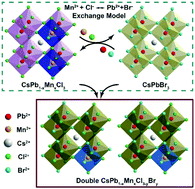
The emission colors of cesium lead halide perovskite nanocrystals (NCs) can be controlled by dynamic ion exchange and Mn2+ doping. Herein we report a novel strategy for the synthesis of Cs(PbxMn1−x)(ClyBr1−y)3 NCs via a post-synthetic cation–anion cosubstitution exchange reaction to tailor their optical properties in a wide range. Colloidal CsPbBr3 and CsPb1−xMnxCl3 NCs are firstly prepared, separately, and then mixed together in hexane solution. The Pb2+ and Br− ions in the CsPbBr3 NCs are simultaneously exchanged for the Mn2+ and Cl− ions in the CsPb1−xMnxCl3 NCs, resulting in homogeneous Cs(PbxMn1−x)(ClyBr1−y)3, with preservation of the shape and crystal structure of the initial NCs. This in situ ion exchange method avoids the degradation of photoluminescence originating from the additional purification process of the NCs, and in situ Mn2+ substitution also greatly enhances the emission intensity and quantum yield of the as-obtained NCs, which is found to be related to the bound exciton effect.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...