Catalyst-free one-step synthesis of ortho-tetraaryl perylene diimides for efficient OPV non-fullerene acceptors†
Abstract
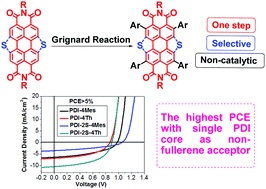
ortho-Functionalized perylene diimides (PDIs) display better electronic properties than their bay-substituted counterparts, yet their synthesis requires expensive catalysts. We report the regioselective 2,5,8,11-functionalization of PDI via the less commonly observed 1,4-addition of aryl Grignard reagents. This direct functionalization offers a simple and low-cost method to synthesize desirable ortho-substituted PDIs. Four tetraarylated PDIs are reported along with their electronic and optoelectronic properties. Preliminary organic photovoltaic devices using these molecules as non-fullerene acceptors showed good power conversion efficiencies (PCEs) up to 5%, a new record for an acceptor with a single PDI core.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...