A green and robust solid catalyst facilitating the magnesium sulfite oxidation in the magnesia desulfurization process†
Abstract
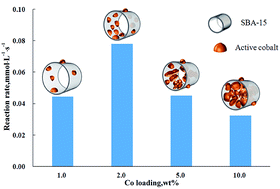
Oxidation of magnesium sulfite is a crucial step in the wet magnesia desulfurization process. In this study, a green and robust solid catalyst, a SBA-15 (SBA, Santa Barbara Amorphous)-supported cobalt catalyst, was developed to promote the oxidation of sulfite. Both Co(III) and Co(II) served as the active sites for the oxidation of sulfite and were mainly located in the inner pore of the SBA-15. The lattice defects formed via the reduction of Co(III) to Co(II) and the dispersion of cobalt played an important role in the catalytic activity. Excess loadings of cobalt resulted in the blockage of the pore and hence decreased the catalytic activity of the catalyst. The catalytic mechanism of sulfite oxidation by this solid catalyst was also proposed. Sulfite was oxidized by the active site, such as Co(III) and Co(II), and the spent catalyst was in situ regenerated by oxygen. Therefore, the diffusion of oxygen into the pore is important to sustain the catalytic activity of the catalyst. Even after four cycles of catalyst reclamation, the catalytic activity was still comparable with that of the aqueous Co2+ counterpart, indicating that the catalyst is robust and efficient. In addition, no leakage of cobalt from the catalyst was detected, implying that it is a green catalyst without risks of secondary pollution. This study is expected to aid in the development of a green and robust catalyst for reclaiming the desulfurization byproduct and downsizing the absorber.


 Please wait while we load your content...
Please wait while we load your content...