Thermodynamics and phase behavior of acid-tethered block copolymers with ionic liquids
Abstract
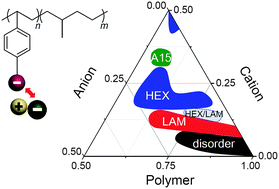
We investigate the phase behavior of acid-tethered block copolymers with and without ionic liquids. Two phosphonated block copolymers and their sulfonated analogs were synthesized by fine-tuning the degree of polymerization and the acid content. The block copolymers carrying acid groups with ionic liquids exhibited rich phase sequences, i.e., disorder–lamellae (LAM), gyroid–LAM, gyroid–hexagonal cylinder (HEX), and gyroid–A15 lattice, and the cation/anion ratio in the ionic liquid exerted profound effects on the segregation strength and topology of the self-assembled structures. Additionally, using ionic liquids with excessive cation content was found to enhance the effective Flory–Huggins interaction parameter, χeff, of the samples. However, as the anion content of the ionic liquids increased the segregation strength decreased. This is attributed to the packing frustration accompanied by the prevailing repulsive electrostatic interactions of the anions in the ionic liquid and the polymer matrix. As the hydrophobicity of the ionic liquids increased, well-defined ordered phases emerged in the phosphonated block copolymers with increased anion content, contrary to the disordered phases of the sulfonated samples. Thus, the balance between solvation energy of the anions and the electrostatic interactions is a key determinant of the thermodynamics of acid-tethered block copolymers containing ionic liquids.
- This article is part of the themed collection: Soft Matter Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...