Laccase–natural mediator systems for “green” synthesis of phenolic monomers from alkali lignin†
Abstract
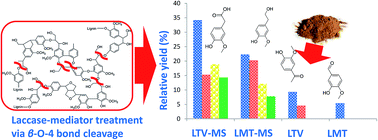
Being a major byproduct of pulp and paper industry, lignin has attracted attention as a source of high-value organic chemicals, e.g. phenolic monomers that can be produced by lignin enzymatic treatment. In this study, the alkali lignin was treated by the laccase of Trametes versicolor (LTV) and laccase of Myceliophthora thermophila (LMT) laccases with or without natural mediator methyl syringate (MS). After treatment, the lignin pore volume has been increased by 66% and 167% for LTV–MS and LMT–MS systems, respectively. The mass balance analysis confirms that the 5 wt% LTV–5 wt% MS (LTV–MS (5, 5)) system produces 40 wt% more of the organic compounds than the 5 wt% LMT–5 wt% MS (LMT–MS (5, 5)) system, thus demonstrating higher efficiency of the LTV–MS toward lignin decomposition. The gas chromatography-mass spectroscopy (GCMS) analysis indicates that after lignin treatment with LTV–MS more phenolic products are produced in comparison to LMT–MS, among them 3-hydroxy-4-methoxy-benzaldehyde has the highest relative yield of 34.7 and 23.8 wt% for LTV–MS and LMT–MS systems, respectively. Pyrolysis-GCMS (pyr-GCMS) of the solid-state lignin residue after its treatment with laccase–mediator system (LMS) confirms significant enrichment of the solid-state lignin surface with phenolic groups. The total organic carbon (TOC) analysis shows that 38.76 mg L−1 of the organic carbon is produced by the LTV–MS (5, 5) system. A decrease in the decomposition temperature by 4 °C for the lignin sample treated by the LTV–MS (in comparison to LMT–MS) obtained from the thermogravimetric (TGA) analysis confirms superior performance of the LTV–MS vs. LMT–MS system. A comparative study of the enzymatically active systems with two laccases and a natural mediator applied to the alkali lignin has been performed. This study highlights an important role of the laccase in combination with a mediator methyl syringate for production of high-value phenolic monomers.


 Please wait while we load your content...
Please wait while we load your content...