Abstract
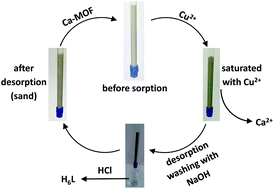
A new Ca2+ two-dimensional framework, namely [Ca(H4L)(DMA)2]·2DMA (Ca-MOF) was obtained from the initial use of N,N′-bis(2,4-dicarboxyphenyl)-oxalamide (H6L). We discovered that this Ca-MOF is capable of exchanging the Ca2+ ions by Cu2+ almost quantitatively in a matter of seconds in aqueous solution. The highly efficient Cu2+ sorption properties exhibited by the Ca-MOF were investigated in detail via batch ion-exchange studies. In addition, the Ca-MOF was utilized as a stationary phase in an ion-exchange column for Cu2+ removal from aqueous media. Furthermore, we were able to recycle the most expensive part of the framework, i.e. the H6L, by treating the column with dilute NaOH followed by HCl. The Ca-MOF represents the first example of any MOF capable of exchanging its constituent metal ions that is shown to be a highly effective ion-exchange material, thus opening a new window in the exploitation of MOFs for ion-exchange applications.
- This article is part of the themed collection: In honour of Mercouri G. Kanatzidis for his contributions to Inorganic Chemistry for over 30 years


 Please wait while we load your content...
Please wait while we load your content...