Pd-Catalyzed regioselective C–H halogenation of quinazolinones and benzoxazinones†
Abstract
A Pd-catalyzed ortho-selective halogenation of benzoxazinone and quinazolinone scaffolds has been described employing N-halosuccinimide as both a halogen source and an oxidant reagent via C–H bond activation. This transformation shows high chemo- and regioselectivities and demonstrates a broad range of benzoxazinone and quinazolinone substrates with different functional groups and has been scaled up to the gram level.
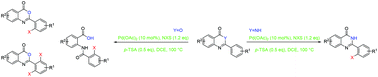
- This article is part of the themed collection: Celebrating excellence in research: women of organic chemistry


 Please wait while we load your content...
Please wait while we load your content...