Design and synthesis of cenocladamide analogues and their evaluation against breast cancer cell lines†‡
Abstract
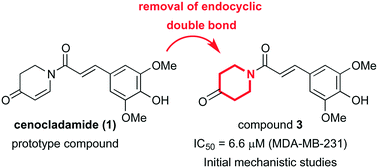
This work describes the total synthesis of the alkaloid cenocladamide and a concise library of nine structural analogues aiming at their evaluation against the breast cancer cell line MDA-MB-231. The most promising compound (3; IC50 = 6.6 μM) was also evaluated in a panel of seven breast cancer cell lines and two non-tumorigenic cell lines. We further conducted an initial investigation on the mechanism of action of analogue 3, which lacks the endocyclic double bond when compared to cenocladamide. The present study presents the discovery of a cenocladamide analogue with interesting cytotoxic activity, which could be useful for further optimization towards new chemotherapeutic agents for breast cancer treatment.

 Please wait while we load your content...
Please wait while we load your content...