New insights into polyene macrolide biosynthesis in Couchioplanes caeruleus†
Abstract
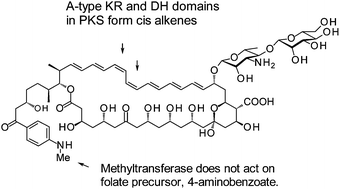
Couchioplanes caeruleus DSM43634 synthesises 67–121C, an aromatic heptaene macrolide that contains a mannosyl-mycosaminyl disaccharide. An improved draft genome sequence was used to obtain the biosynthetic gene cluster for this antifungal. Bioinformatic analysis of the polyketide synthase indicated that extension modules 7 and 8 contain A-type ketoreductase and dehydratase domains. These modules are therefore predicted to form cis double bonds. The deduced stereostructure of the 67–121C macrolactone is identical to that experimentally determined for the partricin subgroup of aromatic heptaenes. Some of these polyenes are N-methylated on the aminoacetophenone moiety. The C. caeruleus AceS protein was shown to methylate 4-aminoacetophenone and esters of 4-aminobenzoate, but not 4-aminobenzoate. This suggests that the substrate specificity of AceS prevents it from interfering with folate biosynthesis. The methyltransferase should be valuable for chemoenzymatic alkylation of compounds that contain aminobenzoyl moieties.


 Please wait while we load your content...
Please wait while we load your content...