Demixing the miscible liquids: toward biphasic battery electrolytes based on the kosmotropic effect†
Abstract
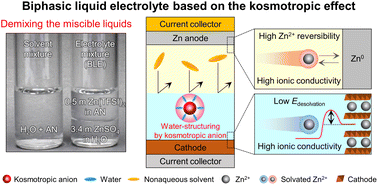
Exploring new electrolyte chemistry beyond conventional single-phase battery electrolytes is needed to fulfill the heterogeneous requirements of anodes and cathodes. Here, we report a biphasic liquid electrolyte (BLE) based on the kosmotropic effect. The key underlying technology for the BLE is phase separation of its electrolyte couples using a principle of “demixing the miscible liquids”. Kosmotropic/chaotropic anions affect the ion coordination structures and the intermolecular interactions of electrolyte couples, enabling on-demand control of their immiscibility/miscibility. Despite the intrinsic miscibility of water (in aqueous electrolytes) and acetonitrile (in nonaqueous electrolytes), the structural change of the aqueous electrolyte induced by kosmotropic anions allows demixing with the nonaqueous electrolyte. The resultant BLE facilitates redox kinetics at cathodes and Zn plating/stripping cyclability at anodes. Consequently, the BLE enables Zn-metal full cells to exhibit a long cyclability (86.6% retention after 3500 cycles). Moreover, Zn anode-free full cells with the BLE exhibit a higher energy density (183 W h kg−1) than previously reported Zn batteries.


 Please wait while we load your content...
Please wait while we load your content...