Atom- and step-economical nucleophilic arylation of azaaromatics via electrochemical oxidative cross C–C coupling reactions†
Abstract
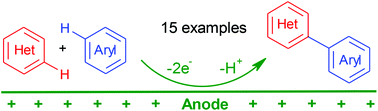
The synthesis of asymmetrical bi(het)aryls through direct functionalization of the C(sp2)–H bond in azaaromatics with fragments of (hetero)aromatic nucleophiles has first been carried out under electrochemical oxidative conditions. This versatile method for C–C bond formation between two aryl fragments can be realized under very mild potential-controlled oxidative conditions, and it does require neither incorporation of any halogen atoms or other leaving groups, nor the use of metal catalysts. The use of the electrochemical SHN methodology for modification of azaaromatic compounds has first been demonstrated.
- This article is part of the themed collection: 2017 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...