Soy peptide aggregates formed during hydrolysis reduced protein extraction without decreasing their nutritional value
Abstract
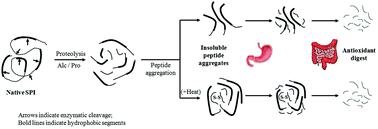
Upon enzymatic hydrolysis, soy protein isolates showed a strong tendency to aggregate, presenting a significant loss of valuable proteins. This study mainly focused on the large insoluble aggregates formed during proteolysis, and the influence of heating was further explored for a better understanding of the mechanism involved. The results from SDS-PAGE and amino acid analysis clearly showed that the insoluble aggregates formed upon hydrolysis were aggregated peptides, mainly attributed to the hydrophobic interactions between peptides with hydrophobic amino acids (Val, Ala, Leu, Ile, Tyr, Phe, and Pro) and sulfur-containing (Met and Cys) residues. Heating of the hydrolysates further enhanced the peptide–protein interactions through hydrophobic forces and disulfide bonds, accelerating the aggregation, where fractions from the basic subunits of glycinin were particularly involved. Furthermore, taking into consideration the fact that aggregates had a high proportion of essential amino acids, the in vitro digestion properties of the aggregates were also investigated. Interestingly, the relatively pepsin-resistant aggregates showed a high degradability toward pancreatin, releasing low molecular weight peptides possessing a higher proportion of antioxidative amino acids, which therefore had a better antioxidant activity. These results indicated a potential use of the insoluble peptide aggregates as protein supplements or active delivery systems for human consumption.
- This article is part of the themed collection: International Symposium on Bioactive Peptides


 Please wait while we load your content...
Please wait while we load your content...