Electrocatalytic hydrogenation of pyridinium enabled by surface proton transfer reactions†
Abstract
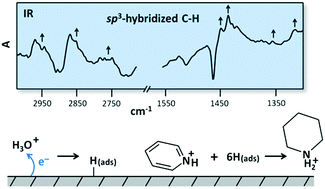
It is observed that pyridinium is hydrogenated at Pt electrodes in electrochemical conditions consistent with those previously shown to yield selective reduction of carbon dioxide to methanol and formic acid. The hydrogenation proceeds through a heterogeneous reaction with chemisorbed hydrogen, which originates from one-electron surface proton transfer reactions. Electrochemical methods are used to show that pyridinium adsorbs on the Pt surface, consistent with the proposed heterogeneous reaction mechanism. From this first observation of the electrochemical generation of a stable hydrogenated piperidinium-like near-surface species it logically follows that dihydropyridinium, the protonated form of the previously-proposed hydride-shuttling reduction catalyst, must transiently exist under these conditions near the Pt surface in the presence of carbon dioxide. Therefore partially hydrogenated heterocycles remain strong candidates for catalytically active species that enable selective carbon dioxide reduction. More generally, the observed mild potentials required for electrocatalytic hydrogenation of stable organics implies that engineered transfer hydrogenations involving organic adsorbates can be a viable approach for achieving selective carbon dioxide reduction to fuels.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...