Can an ammonium-based room temperature ionic liquid counteract the urea-induced denaturation of a small peptide?†
Abstract
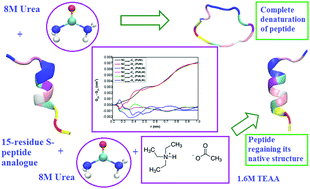
The folding/unfolding equilibrium of proteins in aqueous medium can be altered by adding small organic molecules generally termed as co-solvents. Denaturants such as urea are instrumental in the unfolding of proteins while protecting osmolytes favour the folded ensemble. Recently, room temperature ionic liquids (ILs) have been shown to counteract the deleterious effect of urea on proteins. In this paper, using atomistic molecular dynamics we show that a ternary mixture containing a particular ammonium-based IL, triethylammonium acetate (TEAA), and urea (in 1 : 5 molar ratio) helps a small 15-residue S-peptide analogue regain most of its native structure, whereas a binary aqueous mixture containing a large amount of urea alone completely distorts it. Our simulations show that the denaturant urea directly interacts with the peptide backbone in the binary mixture while for the ternary mixture both urea as well as the IL are preferentially excluded from the peptide surface.
- This article is part of the themed collection: New Frontiers in Indian Research


 Please wait while we load your content...
Please wait while we load your content...