Formal total synthesis of the akuammiline alkaloid (+)-strictamine†
Abstract
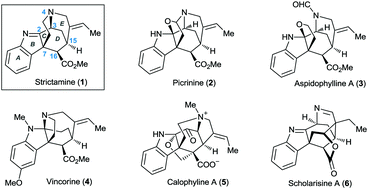
An asymmetric formal total synthesis of the akuammiline alkaloid (+)-strictamine is reported. The key features of the synthesis include a Friedel–Crafts cyclization to form the D-ring and the critical C7 all-carbon quaternary carbon centre, as well as an aza-1,6-conjugate addition to assemble the 2-azabicyclo[3.3.1]nonane system.


 Please wait while we load your content...
Please wait while we load your content...