Lewis acid catalyzed cascade annulation of alkynols with α-ketoesters: a facile access to γ-spiroketal-γ-lactones†
Abstract
A novel Lewis acid catalyzed intermolecular cascade annulation of alkynols with α-ketoesters has been developed. This simple and efficient cascade annulation proceeds through a 5-exo-dig cyclization of alkynols followed by annulation with α-ketoester to provide a wide variety of unsaturated γ-spiroketal-γ-lactones (1,6-dioxaspiro[4.4]non-3-en-2-ones) related to many natural products.
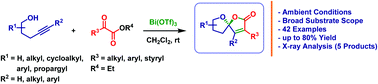


 Please wait while we load your content...
Please wait while we load your content...