Synthesis of enaminones via copper-catalyzed decarboxylative coupling reaction under redox-neutral conditions†
Abstract
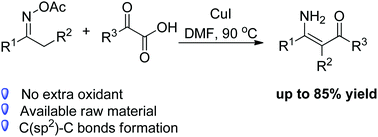
A novel copper-catalyzed C(sp3)–H oxidative functionalization of aromatic oxime acetates with α-oxocarboxylic acids was reported. This process involved N–O/C–C bond cleavages and C–C bond formations to furnish substituted enaminones under redox-neutral conditions. The oxime acetates served as both reactants and internal oxidants. Furthermore, this transformation also features good functional group tolerance and needs no ligands or additional bases.
- This article is part of the themed collection: Most downloaded articles of 2017: Catalysis


 Please wait while we load your content...
Please wait while we load your content...