Assay for Listeria monocytogenes cells in whole blood using isotachophoresis and recombinase polymerase amplification†
Abstract
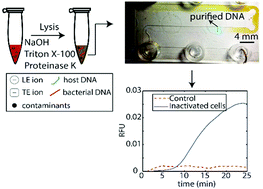
We present a new approach which enables lysis, extraction, and detection of inactivated Listeria monocytogenes cells from blood using isotachophoresis (ITP) and recombinase polymerase amplification (RPA). We use an ITP-compatible alkaline and proteinase K approach for rapid and effective lysis. We then perform ITP purification to separate bacterial DNA from whole blood contaminants using a microfluidic device that processes 25 μL sample volume. Lysis, mixing, dispensing, and on-chip ITP purification are completed in a total of less than 50 min. We transfer extracted DNA directly into RPA master mix for isothermal incubation and detection, an additional 25 min. We first validate our assay in the detection of purified genomic DNA spiked into whole blood, and demonstrate a limit of detection of 16.7 fg μL−1 genomic DNA, the equivalent of 5 × 103 cells per mL. We then show detection of chemically-inactivated L. monocytogenes cells spiked into whole blood, and demonstrate a limit of detection of 2 × 104 cells per mL. Lastly, we show preliminary experimental data demonstrating the feasibility of the integration of ITP purification with RPA detection on a microfluidic chip. Our results suggest that ITP purification is compatible with RPA detection, and has potential to extend the applicability of RPA to whole blood.

 Please wait while we load your content...
Please wait while we load your content...