Molecular insights into the allosteric coupling mechanism between an agonist and two different transducers for μ-opioid receptors†
Abstract
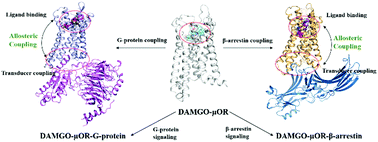
G protein-coupled receptors (GPCRs) as the most important class of pharmacological targets regulate G-protein and β-arrestin-mediated signaling through allosteric interplay, which are responsible for different biochemical and physiological actions like therapeutic efficacy and side effects. However, the allosteric mechanism underlying preferentially recruiting one transducer versus the other has been poorly understood, limiting drug design. Motivated by this issue, we utilize accelerated molecular dynamics simulation coupled with potential of mean force (PMF), molecular mechanics Poisson Boltzmann surface area (MM/PBSA) and protein structure network (PSN) to study two ternary complex systems of a representative class A GPCR (μ-opioid receptor (μOR)) bound by an agonist and one specific transducer (G-protein and β-arrestin). The results show that no significant difference exists in the whole structure of μOR between two transducer couplings, but displays transducer-dependent changes in the intracellular binding region of μOR, where the β-arrestin coupling results in a narrower crevice with TM7 inward movement compared with the G-protein. In addition, both the G-protein and β-arrestin coupling can increase the binding affinity of the agonist to the receptor. However, the interactions between the agonist and μOR also exhibit transducer-specific changes, in particular for the interaction with ECL2 that plays an important role in recruiting β-arrestin. The allosteric network analysis further indicates that Y1483.33, F1523.37, F1563.41, N1914.49, T1603.45, Y1062.42, W2936.48, F2896.44, I2485.54 and Y2525.58 play important roles in equally activating G-protein and β-arrestin. In contrast, M1613.46 and R1653.50 devote important contributions to preferentially recruit G-protein while D1643.49 and R179ICL2 are revealed to be important for selectively activating β-arrestin. The observations provide useful information for understanding the biased activation mechanism.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...