Study on the selective oxidation of methane over highly dispersed molybdenum-incorporated KIT-6 catalysts†
Abstract
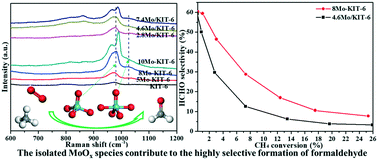
A series of molybdenum-incorporated mesoporous silica (Mo–KIT-6) catalysts were successfully synthesized by a one-pot hydrothermal synthesis method, and were applied in the selective oxidation of methane to formaldehyde using oxygen as an oxidizing agent under atmospheric pressure. Comparatively, the corresponding supported catalysts (Mo/KIT-6) were prepared by incipient-wetness-impregnation method. The results of the small angle XRD, nitrogen adsorption/desorption isotherms, UV-vis, H2-TPR and UV-Raman spectroscopy characterization combined with the catalytic activity tests demonstrated that molybdenum atoms were inserted into the framework of the mesoporous materials for the Mo–KIT-6 catalysts and the highly dispersed Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds dominantly existed, which were responsible for the efficient selective formation of formaldehyde. However, for Mo/KIT-6 catalysts, the molybdenum oxide species were mainly loaded on the surface or inside the outer pore channels of the support and abundant emergence of the Mo–O–Mo bond played a major role in the activation of methane to COx. Furthermore, with equivalent molybdenum content, the methane selective oxidation performance of 8Mo–KIT-6 was obviously better than that of 4.6Mo/KIT-6, and the formaldehyde yield (2.1%) of 8Mo–KIT-6 was 2.3 times as much as that (0.9%) of 4.6Mo/KIT-6. In situ and operando UV-Raman results demonstrated that the structures of the MoOx active sites have a strong effect on the formation and elimination of carbon deposition during the separated redox reaction with methane and O2, respectively. The polymerized MoOx active sites are favorable for the formation of graphitic carbon (G), which is called ordered carbon, while the isolated MoOx active sites are favorable for the formation of disordered carbon (D). The reduced highly dispersed MoOx active sites incorporated in the framework of silica are more easily reoxidized than those on the supported catalysts.
O bonds dominantly existed, which were responsible for the efficient selective formation of formaldehyde. However, for Mo/KIT-6 catalysts, the molybdenum oxide species were mainly loaded on the surface or inside the outer pore channels of the support and abundant emergence of the Mo–O–Mo bond played a major role in the activation of methane to COx. Furthermore, with equivalent molybdenum content, the methane selective oxidation performance of 8Mo–KIT-6 was obviously better than that of 4.6Mo/KIT-6, and the formaldehyde yield (2.1%) of 8Mo–KIT-6 was 2.3 times as much as that (0.9%) of 4.6Mo/KIT-6. In situ and operando UV-Raman results demonstrated that the structures of the MoOx active sites have a strong effect on the formation and elimination of carbon deposition during the separated redox reaction with methane and O2, respectively. The polymerized MoOx active sites are favorable for the formation of graphitic carbon (G), which is called ordered carbon, while the isolated MoOx active sites are favorable for the formation of disordered carbon (D). The reduced highly dispersed MoOx active sites incorporated in the framework of silica are more easily reoxidized than those on the supported catalysts.


 Please wait while we load your content...
Please wait while we load your content...