Density functional theory and experimental studies of caffeic acid adsorption on zinc oxide and titanium dioxide nanoparticles
Abstract
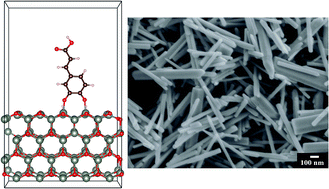
The outstanding adsorption properties of proteins, containing catecholic amino acid, L-3,4-dihydroxyphenylalanine (DOPA), and recent advances in nanoparticle functionalization using molecules from the catechol family have generated interest in the investigation of catechol adsorption and applications of catecholates in nanotechnology. Caffeic acid (CA) is the closest molecular analogue of DOPA. Density functional theory has been applied for the modelling of CA adsorption on the surface of ZnO and TiO2. Different adsorption modes have been investigated and corresponding adsorption energies were evaluated. According to the calculated energies, the adsorption of CA is energetically favourable at both surfaces with a stronger affinity to TiO2. The results of theoretical studies were supported by experimental investigations of CA adsorption. The use of CA as a dispersant for hydrothermal synthesis of ZnO allowed for the fabrication of ZnO nanorods with reduced size and increased aspect ratio. The CA, adsorbed during the hydrothermal synthesis on ZnO nanorods, allowed for their electrosteric dispersion and the electrophoretic deposition (EPD) of ZnO films from stable colloidal suspensions. In another strategy, CA was added as a dispersant for the dispersion of TiO2 nanorods and the EPD of TiO2 films. The advantages of catecholates for the synthesis of nanoparticles and fabrication of thin films are discussed.

 Please wait while we load your content...
Please wait while we load your content...