Electrochemical oxidative cyclization of alkenes, boronic acids, and dichalcogenides to access chalcogenated boronic esters and 1,3-diols†
Abstract
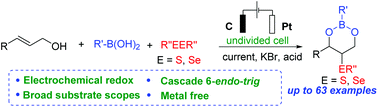
A sustainable, environmentally benign electrochemical oxidative three-component cyclization of allylic alcohols, boronic acids, and dichalcogenides under metal-free and oxidant-free conditions has been developed, which provides an efficient approach for the formation of C–Se/S and C–O bonds together. A series of chalcogenated boronic esters were afforded with a broad substrate scope through a clean electrochemical system. The resulting chalcogenated cyclic boronic esters were easily converted to chalcogenated 1,3-diols in good yields under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...