Photophysical properties of push–pull 8-aryl-deoxyguanosine probes within duplex and G-quadruplex structures†
Abstract
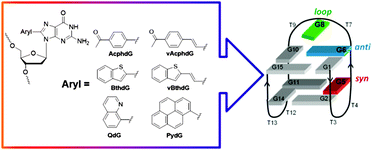
In this study, we outline the structural and photophysical properties of donor–acceptor (D–A) 8-aryl-2′-deoxyguanosine (8aryldG) probes within duplex and G-quadruplex (GQ) structures produced by the thrombin binding aptamer (TBA, 5′-GGTTG5G6TG8TGGTTGG). The probes vary in 8-aryl ring size, degree of twist angle between the aryl ring and nucleobase component, degree of acceptor character, and nature of visibly emissive charge transfer (CT) states. Probes with the aryl ring directly attached to the nucleobase favor the syn-conformation and strongly destabilize the duplex structure, as measured by UV thermal melting experiments. However, these probes can stabilize the antiparallel GQ produced by TBA when inserted into the G-tetrad at the syn-G5 position, and strongly decrease GQ stability when inserted at the anti-G6 position. Nucleoside probes with the aryl ring separated from the nucleobase by a vinyl linker favor the anti-conformation. Furthermore, the nature of the aryl group dictates an ability of these probes to be accomodated within the GQ at the anti-G6 position. 8AryldG probes that favor planar CT states (CTP) exhibit bright emission in both duplex and GQ structures, but lack fluorescence sensitivity to changes in the microenvironment. In contrast, probes that afford twisted CT states (CTT) exhibit weak fluorescence in duplex and GQ structures in water, but display fluorescence signalling capability for monitoring GQ formation in a crowded environment.
- This article is part of the themed collection: Shape-Responsive Fluorophores

 Please wait while we load your content...
Please wait while we load your content...