A silver-inserted zinc rhodium oxide and bismuth vanadium oxide heterojunction photocatalyst for overall pure-water splitting under red light†
Abstract
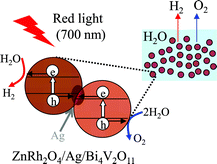
We have prepared a solid-state heterojunction photocatalyst, in which zinc rhodium oxide (ZnRh2O4) and bismuth vanadium oxide (Bi4V2O11) as hydrogen (H2) and oxygen (O2) evolution photocatalysts, respectively, were connected with silver (Ag, ZnRh2O4/Ag/Bi4V2O11). ZnRh2O4/Ag/Bi4V2O11 was able to photocatalyze overall pure-water splitting under red-light irradiation with a wavelength of 700 nm. On the basis of the analogy with a solid-state heterojunction photocatalyst composed of ZnRh2O4, defective silver antimonate (Ag1−xSbO3−y), and Ag (R. Kobayashi et al., J. Phys. Chem. C, 2014, 118, 22450–22456), we consider that the overall water-splitting performance of the ZnRh2O4/Ag/Bi4V2O11 photocatalyst was derived from the photoproduced holes that were generated in the valence band (VB) of Bi4V2O11 contributing to O2 liberation and the photoexcited electrons that were generated in the conduction band (CB) of ZnRh2O4 contributing to H2 liberation. Importantly, Ag possibly acts as a solid-state electron mediator for the transfer of electrons from the CB of Bi4V2O11 to the VB of ZnRh2O4.
- This article is part of the themed collection: Water splitting and photocatalysis

 Please wait while we load your content...
Please wait while we load your content...