Multihierarchical electrodes based on titanate nanotubes and zinc oxide nanorods for photoelectrochemical water splitting†
Abstract
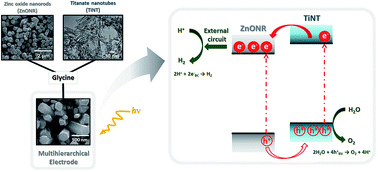
Studies involving water splitting to form hydrogen and oxygen have attracted attention because H2 is considered the fuel of the future. Photoelectrocatalysts have been widely used for this application, and several metal oxides can be applied as catalysts. Among them, we highlight zinc oxide nanorods (ZnONRs) and titanate nanotubes (TiNTs); however, their individual nanostructures exhibit disadvantages. For example, ZnONRs show rapid recombination of the photogenerated charges, and TiNTs give rise to randomly oriented films; these disadvantages limit their application as photoanodes. In this study, for the first time, we present a new class of multihierarchical electrodes based on TiNT-decorated ZnONR films that exhibited superior results to the individual species. The TiNTs are homogenously dispersed over the surface of the rods without forming agglomerates, giving rise to a heterojunction that exhibits lower recombination rates. It was found that the results are better when the contents of TiNTs in the electrode are higher; thus, glycine was successfully used as a bridge to link both of the structures, increasing the amount of TiNTs decorating the rods. As a result, the photocurrent generated with these multihierarchical electrodes is higher than that obtained with pure ZnONR electrodes (0.9 mA cm−2 and 0.45 mA cm−2 at 1.23 VRHE, respectively), and the electrode potentials for O2 evolution are lower than those observed for pure TiNT electrodes (0 V and 0.8 V vs. ERHE, respectively). The IPCE values are also higher for the multihierarchical electrodes.
- This article is part of the themed collection: SBQ-RSC: Celebrating UK-Brazil collaborations

 Please wait while we load your content...
Please wait while we load your content...