Periodate – an alternative oxidant for testing potential water oxidation catalysts
Abstract
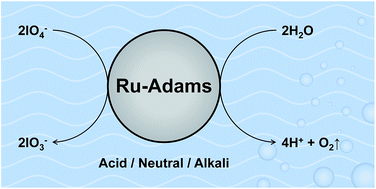
The redox catalyst ruthenium dioxide, prepared via the Adams technique, i.e. Ru(Adams), is used as a water oxidation catalyst using the oxidants (i) Ce(IV) in 0.5 M H2SO4 and (ii) periodate in 0.5 M H2SO4, water and 0.1 M KOH. Like Ce(IV), periodate is a very strong oxidant that is able to oxidise water to oxygen and can be readily monitored spectrophotometrically at 280 nm, compared with 430 nm for Ce(IV). More importantly, unlike Ce(IV), which is unstable towards hydrolysis above pH 1, periodate is stable in acid, water and strong alkali. A spectrophotometric study of the kinetics of periodate reduction, and concomitant oxidation of water to O2, reveals that in the presence of a suitable redox catalyst, Ru(Adams) in this work, periodate is able to effect the stoichiometric oxidation of water, with a turnover number > 48. In just water, the kinetics of the latter reaction appear diffusion-controlled, due to the large thermodynamic driving force, a measure of which is the difference in redox potential, i.e. ΔE = 423 mV. As this difference is decreased, ΔE = 396 mV in acid and 290 mV in strong alkali (0.1 M KOH), the kinetics become increasingly activation-controlled and slower. These findings are discussed briefly with regard to the possible use of (i) periodate as an alternative oxidant in the rapid screening of new potential water oxidation catalyst material powders that are stable only under near neutral and/or alkaline conditions, and (ii) Ru(Adams) as a benchmark catalyst.
- This article is part of the themed collection: Water splitting and photocatalysis

 Please wait while we load your content...
Please wait while we load your content...