An investigation on the aqueous-phase hydrodeoxygenation of various methoxy-substituted lignin monomers on Pd/C and HZSM-5 catalysts†
Abstract
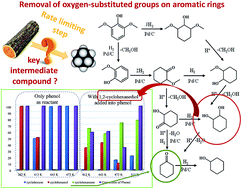
Aqueous phase catalytic upgrading of lignin monomers to hydrocarbons via hydrodeoxygenation (HDO) has been explored using a combination of Pd/C and HZSM-5 catalysts under 2 MPa of H2 (ambient temperature). Model monomers with varying numbers of methoxy groups, including phenol, anisole, guaiacol and 2,6-dimethoxy-phenol, were chosen as lignin model compounds. Mechanistic studies revealed cascade and parallel reaction pathways via hydrogenation and dehydration (hydrolysis) processes, which were catalyzed by Pd/C and HZSM-5, respectively. Hydrogenation was preferred at lower temperature, whereas higher temperature was favorable for the removal of oxygen-containing functional groups. The effect of methoxy groups on the HDO of these monomers was also investigated systematically. Basically, the conversion of multi-substituted monomers was tougher than that of mono-substituted ones, due to steric constraint and the inhibition of the electron-donating hydroxyl group. The selectivities to cyclohexane from phenol and anisole were improved significantly by increasing the temperature to 413 K. However, cyclohexanone was preferably produced over cyclohexane when using model compounds with multi-substituents (guaiacol and 2,6-dimethoxy-phenol), even at temperatures above 513 K. Comparative experiments were also conducted on the HDO of 1,2-cyclohexanediol with or without the presence of phenol, which clearly suggested that the further HDO of guaiacol and 2,6-dimethoxy-phenol was inhibited, probably due to the strong interactions between cyclohexanediol molecules and Brønsted acid sites.

 Please wait while we load your content...
Please wait while we load your content...