Experimental and theoretical study of intramolecular O⋯O interaction in structurally rigid β-keto carboxylic esters†
Abstract
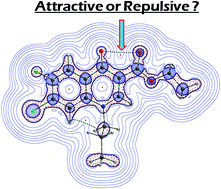
Here we report the crystal structures of quinolone carboxylate and bisethoxycarbonylvinylaniline derivates containing an O⋯O distance shorter than the sum of their van der Waals radii, which can be ascribed to their steric demand per se, which provide unequivocal evidence of intramolecular 1,5-closed shell type interaction. Theoretical studies including Quantum Theory of Atoms in Molecules (QTAIM) and Natural Bond Orbital (NBO) analysis are employed to characterize the nature of the closed shell O⋯O interaction. We found that the lone pair electrons on the interacting oxygens undergo stabilization due to negative hyperconjugation and maintains the otherwise repulsive O⋯O close contact.
- This article is part of the themed collection: Editors Collection for RSC Advances - India

 Please wait while we load your content...
Please wait while we load your content...