Green and practical transition metal-free one-pot conversion of substituted benzoic acids to anilines using tosyl azide†
Abstract
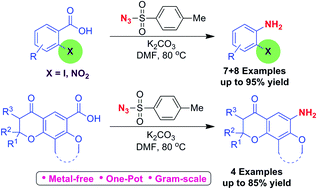
A simple one-pot procedure for conversion of 2-iodo/2-nitro benzoic acids and dihydropyranone fused benzoic acids into corresponding anilines, using tosyl azide, under transition metal-free conditions is explained. TsN3, is one of the more stable azides, much cheaper and easy to handle. This method is environmentally benign, adoptable for large scale preparation. Different factors such as steric and electronic effect of 2-iodo/2-nitro substituents, and the effect of conformational constraints of the fused dihydropyranone ring on co-planarity of the aromatic ring should contribute to the success of this reaction. Tosyl nitrene was found to be involved in the decarboxylation reaction. As this method is simple, scalable and requires only simple reagents we expect this method to find widespread application in organic synthesis.
- This article is part of the themed collection: Celebrating the 75th Birthday of Professor Barry Trost

 Please wait while we load your content...
Please wait while we load your content...