Pd(ii)-catalyzed direct functionalization of C–H bonds of benzamides for synthesis of 1,1-difluoro-1-alkenes†
Abstract
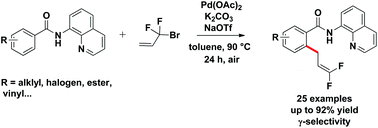
Pd-catalyzed direct difluoroallylation of C–H bonds of benzamides with readily available Rf-Br reagents is reported. The reaction proceeds via Pd-catalyzed C–H activation, followed by reaction of the resulting intermediate with 3-bromo-3,3-difluoropropene (BDFP) in a γ-selective fashion. The C–H functionalization process was characterized by a broad substrate scope as well as an excellent functional-group tolerance.
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers in 2016

 Please wait while we load your content...
Please wait while we load your content...