Potassium-mediated stereochemical assistance to form one indenonaphthacene isomer from rubrene with complementary diastereoselectivity to the acid based protocol†
Abstract
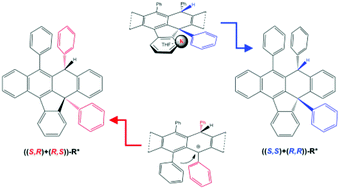
Diastereoselective C–H and C–C bond formation has been assessed from rubrene to form selectively one indenonaphthacene derivative R*, in the presence of metallic potassium stabilised by THF at room temperature. The potassium salt [K(rac-R*)(THF)x] contains R* as anion with a (1S,2S) and (1R,2R) configuration, on the two new chiral carbon centers, which has been unambiguously confirmed by selective 1D NOESY, 1H, 13C-NMR, 1H, 13C-HMBC and polarimetry. The stated chirality could only originate from a face selective intramolecular reaction, which takes place on one side of the original tetracene core. The diastereomeric excess for the potassium salt has been quantified by 1H NMR to be at least 97%. Potassium removal affords the neutral ((S,S)+(R,R))-R*. In contrast, the acid-based protocol furnishes two structural isomers, ((S,R)+(R,S))-R* and rac-R′. The former compound has been isolated pure with a yield of 56% by precipitation. The complementary chirality in R* is a direct consequence of the inter- and intramolecular character for both face-selective processes, along with potassium assistance in the latter case.
- This article is part of the themed collection: Celebrating the 75th Birthday of Professor Barry Trost

 Please wait while we load your content...
Please wait while we load your content...