Structure, phase transition and negative thermal expansion in ammoniated ZrW2O8†
Abstract
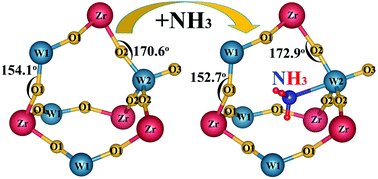
In the present work, we have developed an effective way to control the thermal expansion of ZrW2O8 by the insertion of NH3 into the void of the framework structure. Neutron powder diffraction (NPD) and XPS studies reveal that N is bonding to W in the ammoniated ZrW2O8 and retains the original structure with the space group P213. This kind of ammoniation improves the thermal stability, raised the phase transition temperature about 50 K, and weakens the negative thermal expansion (NTE) from −7.8 × 10−6 K−1 to −2.1 × 10−6 K−1. These behaviors account for bonding of NH3 to the neighboring atoms of ZrW2O8, and hinder the rocking motion of the corner shared polyhedral structure.

 Please wait while we load your content...
Please wait while we load your content...