Hmboff/on as a switchable thiol protecting group for native chemical ligation†
Abstract
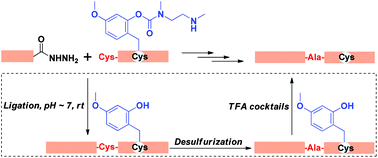
A new thiol protecting group Hmboff/on is described, which has a switchable activity that may be useful in the chemical synthesis of proteins. When placed on the side chain of Cys, Cys(Hmboff) is stable to trifluoroacetic acid (TFA) in the process of solid-phase peptide synthesis. When Cys(Hmboff) is treated with neutral aqueous buffers, it is cleanly converted to acid-labile Cys(Hmbon), which can later be fully deprotected by TFA to generate free Cys. The utility of Cys(Hmboff/on) is demonstrated by the chemical synthesis of an erythropoietin segment, EPO[Cys98–Arg166]-OH through native chemical ligation.
- This article is part of the themed collection: Selective Chemistry with Peptides and Proteins
 Please wait while we load your content...
Please wait while we load your content...