First total synthesis of trehalose containing tetrasaccharides from Mycobacterium smegmatis†
Abstract
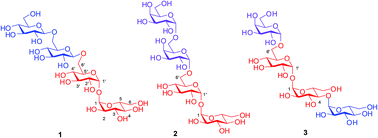
Total synthesis of three important trehalose containing tetrasaccharides isolated from Mycobacterium smegmatis is reported for the first time, using regioselective opening of benzylidene acetals and stereoselective glycosylations as key steps. The 1,2-cis stereoselectivity in the glycosylation reactions was achieved using anchimeric assistance from a remote participating group, steric effects and solvent participation. The synthetic strategy can also be utilized for the assembly of structurally related oligosaccharides from M. tuberculosis.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...