Synthesis of benzofuranyl and indolyl methyl azides by tandem silver-catalyzed cyclization and azidation†
Abstract
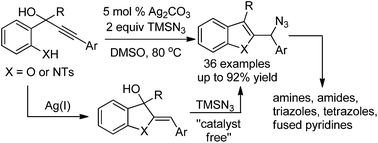
Ag(I)-catalyzed synthesis of 2-azidomethyl benzofurans/indoles from linear and readily available hydroxyl/amino-phenyl propargyl alcohols is described via a highly regioselective C–O and C–N bond formation. Control experiments reveal that the reaction involves the sequential Ag(I)-catalyzed 5-exo-dig cyclization and a catalyst free γ-allylic azidation. The synthetic utility of this method has been demonstrated by using the azidomethyl unit of the above synthesized heterocycles as the base for a variety of other functionalizations, such as triazole-, tetrazole-, amide-, amine-, and pyrido-derivatives.

 Please wait while we load your content...
Please wait while we load your content...