Palladium-catalyzed Suzuki–Miyaura coupling of amides by carbon–nitrogen cleavage: general strategy for amide N–C bond activation†
Abstract
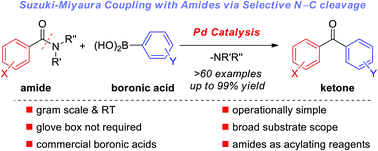
The first palladium-catalyzed Suzuki–Miyaura cross-coupling of amides with boronic acids for the synthesis of ketones by sterically-controlled N–C bond activation is reported. The transformation is characterized by operational simplicity using bench-stable, commercial reagents and catalysts, and a broad substrate scope, including substrates with electron-donating and withdrawing groups on both coupling partners, steric-hindrance, heterocycles, halides, esters and ketones. The scope and limitations are presented in the synthesis of >60 functionalized ketones. Mechanistic studies provide insight into the catalytic cycle of the cross-coupling, including the first experimental evidence for Pd insertion into the amide N–C bond. The synthetic utility is showcased by a gram-scale cross-coupling and cross-coupling at room temperature. Most importantly, this process provides a blueprint for the development of a plethora of metal catalyzed reactions of typically inert amide bonds via acyl-metal intermediates. A unified strategy for amide bond activation to enable metal insertion into N–C amide bond is outlined (
- This article is part of the themed collection: New Talent


 Please wait while we load your content...
Please wait while we load your content...