On the chemical stability of post-lithiated garnet Al-stabilized Li7La3Zr2O12 solid state electrolyte thin films†
Abstract
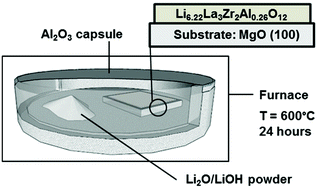
Garnet-based Al-doped Li7La3Zr2O12 has the potential to be used as a solid state electrolyte for future lithium microbattery architectures, due to its relatively high Li+ conductivity and stability against Li. Through this work, a model experiment is presented in which the effect of post-lithiation on phase formation and chemical stability is studied for pulsed laser deposited Al-doped Li7La3Zr2O12 thin films on MgO substrates. We report the implications of the newly suggested post-lithiation route for films with thicknesses between 90 and 380 nm. The phase changes from cubic, to a mix of cubic and tetragonal Li7La3Zr2O12, to a cubic Li7La3Zr2O12 and La2Zr2O7 containing film is accompanied by a reduction in the degree of de-wetting as the thickness increases. This study reveals that the thicker, dense, and continuous films remain predominantly in a mixed phase containing cubic Li7La3Zr2O12 and the lithium free La2Zr2O7 phase whereas the thinner, de-wetted films exhibit improved lithium incorporation resulting in the absence of the lithium free phase. For tuning the electrical conductivity and effective use of these structures in future batteries, understanding this material system is of great importance as the chemical stability of the cubic Li7La3Zr2O12 phase in the thin film system will control its effective use. We report a conductivity of 1.2 × 10−3 S cm−1 at 325 °C for a 380 nm thick solid state electrolyte film on MgO for potential operation in future all solid state battery assemblies.
- This article is part of the themed collection: Electrochemical processes at the nanoscale: from fundamentals to applications

 Please wait while we load your content...
Please wait while we load your content...