Synthesis and mode of action of oligomeric sesquiterpene lactones
Abstract
Covering: up to 2015
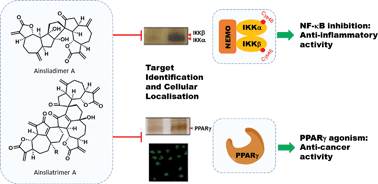
In this highlight we describe two case studies from our laboratory, involving the biomimetic syntheses and the biological mechanism elucidation of the bioactive oligomeric sesquiterpenoids, (+)-ainsliadimer A (4) and (−)-ainsliatrimer A (5). Ainsliadimer A possesses potent anti-inflammatory activity by inhibition of the NF-κB signalling pathway via binding at a previously untargeted allosteric site. (−)-Ainsliatrimer A induces apoptosis in cancer cells by activation of PPARγ. Furthermore, we highlight a new bioorthogonal ligation (TQ-ligation) developed in our laboratory which facilitates the target identification of complex natural products via pre-target fluorescence imaging and affinity chromatography. Generally, this paper will discuss the complete process from total synthesis to biological studies of complex natural products, and from the establishment of new bio-orthogonal chemistry to successful target identification. Our approach provides a systematic and efficient methodology for addressing the challenge of natural product target identification.
- This article is part of the themed collection: Strategies for Cellular Target Identification of Natural Products

 Please wait while we load your content...
Please wait while we load your content...