Synthesis and photophysical characteristics of 2,3,12,13-tetraalkylbacteriochlorins†
Abstract
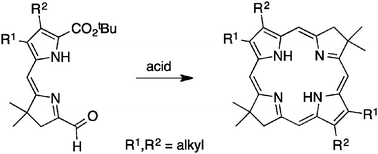
Bacteriochlorins absorb strongly in the near-infrared spectral region and hence are of great interest across the field of photochemistry. The established de novo self-condensation of a dihydrodipyrrin-acetal has afforded stable bacteriochlorins bearing a variety of β-pyrrolic substituents, but the route was incompatible with the presence of two alkyl groups. The lacuna stemmed from the instability of the dialkyl-substituted dihydrodipyrrin-acetal. Here, two dihydrodipyrrin-carboxaldehydes, each bearing a tert-butoxycarbonyl group at the pyrrole α-position, were prepared and found to be stable to routine handling. Each ester-substituted dihydrodipyrrin-carboxaldehyde in acid underwent in situ ester cleavage and ensuing self-condensation to provide the corresponding 2,3,12,13-tetraalkylbacteriochlorin. The alkyl groups examined include methyl and methyl acetate. Such synthetic bacteriochlorins, while previously unknown, provide valuable models of the natural chromophores. The absorption and fluorescence characteristics of the tetraalkylbacteriochlorins are generally typical for this genre of macrocycle. The X-ray structure of the tetramethylbacteriochlorin reveals that the pyrrolinic (reduced) rings are modestly more twisted out-of-plane (torsional angle ∼11°) than those of the nearly planar (torsional angle ∼1°) parent bacteriochlorin that lacks pyrrolic tetraalkyl groups. The resonance Raman spectrum of the tetramethylbacteriochlorin is also far richer in the low- to mid-frequency region than that of the unsubstituted analogue, but like the unsubstituted counterpart, is far sparser in the high-frequency region than that of native bacteriochlorophyll. Access to tetraalkylbacteriochlorins constitutes one step on the path from the most simple, fully unsubstituted bacteriochlorin to the fully decorated, natural bacteriochlorophylls.
- This article is part of the themed collection: Nitrogen Ligands

 Please wait while we load your content...
Please wait while we load your content...