Molecular localization and characterization of multiple binding sites of organic anion transporting polypeptide 2B1 (OATP2B1) as the mechanism for substrate and modulator dependent drug–drug interaction†‡
Abstract
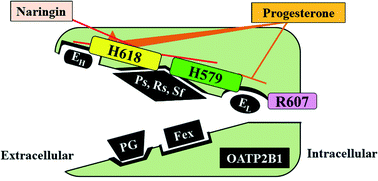
Members of the organic anion transporting polypeptide (OATP) transporter family are involved in drug absorption and disposition, and therefore drug–drug and drug–food interactions at these transporters may have both pharmacological and toxicological effects. Accordingly, a detailed understanding of the substrates and modulators, including inhibitors and stimulators, of the transporters, as well as the resulting interactions, is required. However, this is not straightforward, since OATP transporters have multiple binding sites, which exhibit different selectivity for substrates and modulators. Here, we focused on the characterization of the binding sites of OATP2B1, which contributes to the intestinal absorption and hepatic disposition of drugs. Site-directed mutagenesis studies indicated that histidine residues His579 and His618 are involved in the low- and high-affinity sites for estrone-3-sulfate (E13S) transport, respectively. Uptakes of pravastatin, rosuvastatin and sulfasalazine were decreased by mutations of His579 and His618 of OATP2B1, whereas uptakes of fexofenadine and prostaglandin E2 were not affected. Naringin, an OATP inhibitor, reduced the uptakes of pravastatin, rosuvastatin, sulfasalazine and low-concentration E13S, but not other substrates. In addition, progesterone stimulated the uptake of low-concentration E13S but did not affect or even decreased the uptakes of other substrates. These observations confirm that multiple binding sites are present on OATP2B1, resulting in differential characteristics in the recognition of substrates and modulators. Accordingly, identification of the locations of binding sites on OATP2B1 and their detailed characterization will be necessary to improve the prediction of drug–drug and drug–food interactions on OATP2B1.


 Please wait while we load your content...
Please wait while we load your content...