6-O-Nucleotidyltransferase: an aminoglycoside-modifying enzyme specific for streptomycin/streptidine†
Abstract
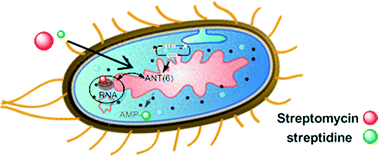
Aminoglycosides are especially useful for the treatment of hospital-acquired infections. The main problem for the application of these antibiotics is the presence of bacterial resistance enzymes, in particular, nucleotidyltransferases (ANTs). These enzymes catalyze the transfer of an adenylyl group from the MgATP complex to different positions of the antibiotic. To understand the mechanisms that lead to antibiotic inactivation, we have performed a comprehensive experimental analysis of one of those enzymes. The 6-O- nucleotidyltransferase enzyme (ANT(6)) from Bacillus subtilis was cloned, overexpressed and purified in E. coli. The kinetic parameters revealed a narrow specificity of the ANT(6) for MgATP/streptomycin as substrates. The binding epitope of the streptomycin recognized by the ANT(6) is the streptidine moiety. Therefore, the use of streptidine as a “decoy acceptor” allows the recovery of the antibiotic activity of streptomycin E. coli cells that are overexpressing the ANT(6).

 Please wait while we load your content...
Please wait while we load your content...