Use of protein-engineered fabrics to identify design rules for integrin ligand clustering in biomaterials†
Abstract
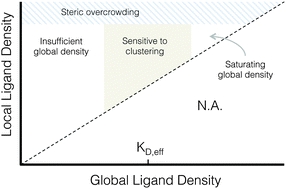
While ligand clustering is known to enhance integrin activation, this insight has been difficult to apply to the design of implantable biomaterials because the local and global ligand densities that enable clustering-enhanced integrin signaling were unpredictable. Here, two general design principles for biomaterial ligand clustering are elucidated. First, clustering ligands enhances integrin-dependent signals when the global ligand density, i.e., the ligand density across the cellular length scale, is near the ligand’s effective dissociation constant (KD,eff). Second, clustering ligands enhances integrin activation when the local ligand density, i.e., the ligand density across the length scale of individual focal adhesions, is less than an overcrowding threshold. To identify these principles, we fabricated a series of elastin-like, electrospun fabrics with independent control over the local (0 to 122 000 ligands μm−2) and global (0 to 71 000 ligand μm−2) densities of an arginine–glycine–aspartate (RGD) ligand. Antibody blocking studies confirmed that human umbilical vein endothelial cell adhesion to these protein-engineered biomaterials was primarily due to αVβ3 integrin binding. Clustering ligands enhanced cell proliferation, focal adhesion number, and focal adhesion kinase expression near the ligand's KD,eff of 12 000 RGD μm−2. Near this global ligand density, cells on ligand-clustered fabrics behaved similarly to cells grown on fabrics with significantly larger global ligand densities but without clustering. However, this enhanced ligand-clustering effect was not observed above a threshold cut-off concentration. At a local ligand density of 122 000 RGD μm−2, cell division, focal adhesion number, and focal adhesion kinase expression were significantly reduced relative to fabrics with identical global ligand density and lesser local ligand densities. Thus, when clustering results in overcrowding of ligands, integrin receptors are no longer able to effectively engage with their target ligands. Together, these two insights into the cellular responses to ligand clustering at the cell–matrix interface may serve as design principles when developing future generations of implantable biomaterials.

 Please wait while we load your content...
Please wait while we load your content...