Functional models of nonheme diiron enzymes: kinetic and computational evidence for the formation of oxoiron(iv) species from peroxo-diiron(iii) complexes, and their reactivity towards phenols and H2O2†
Abstract
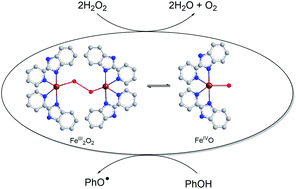
The reactivity of the previously reported peroxo adducts [Fe2(μ-O2)(L1)4(CH3CN)2]2+, and [Fe2(μ-O2)(L2)4(CH3CN)2]2+, (L1 = 2-(2′-pyridyl)benzimidazole and L2 = 2-(2′-pyridyl)-N-methylbenzimidazole) towards H2O2 as catalase mimics, and towards various phenols as functional RNR-R2 mimics, is described. Kinetic, mechanistic and computational studies gave direct evidence for the involvement of the (μ-1,2-peroxo)diiron(III) intermediate in the O–H activation process via formation of low-spin oxoiron(IV) species.
- This article is part of the themed collection: Small Molecule Activation

 Please wait while we load your content...
Please wait while we load your content...