Electrophilic phosphonium cations (EPCs) with perchlorinated-aryl substituents: towards air-stable phosphorus-based Lewis acid catalysts†
Abstract
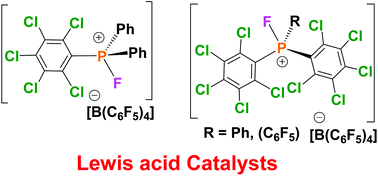
A series of phosphines incorporating (C6Cl5) substituents, Ph2P(C6Cl5) 1, PhP(C6Cl5)22, P(C6Cl5)33 and (C6F5)P(C6Cl5)24 were prepared. In the case of 1, 2 and 4, these were converted to the corresponding aryl-difluorophosphoranes 5–7via reaction with XeF2, whereas reaction of 3 with XeF2 afforded only an inseparable mixture of products. The compounds 5–7 were converted to the fluorophosphonium cations 8–10, whereas the reaction of 3 with Selectfluor afforded (C6Cl5)2POF and (C6Cl5)2. The fluorophosphonium salts showed evidence of improved air stability as well as Lewis acid catalytic activity in hydrodefluorination, hydrosilylation, deoxygenation and dehydrocoupling chemistry.
- This article is part of the themed collection: Small Molecule Activation

 Please wait while we load your content...
Please wait while we load your content...