Introduction of CoCl2·6H2O into Co3O4 for enhancement of hydroxyl radicals and effective charge separation†
Abstract
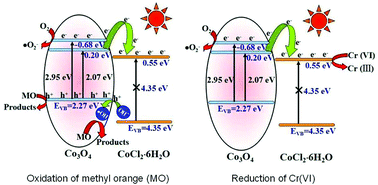
Porous sphere-like tricobalt tetraoxide (Co3O4)–cobalt chloride hydrate (CoCl2·6H2O, CCH) heterojunctions are obtained using a one-step facile solution combustion route. The heterostructure is confirmed by XRD, HRTEM, and XPS measurements. Their photocatalytic performances are evaluated by the degradation of methyl orange (MO) and the reduction removal of Cr(VI) ions under visible light irradiation. The heterojunction containing 81.5 wt% Co3O4 and 18.5 wt% CCH exhibits the highest photocatalytic performance, for which the pseudo-first-order reaction rate constant is 10.0 and 8.7 times that of pure Co3O4 towards MO degradation and Cr(VI) reduction, respectively. This enhancement in activity can be attributed to the effective electron transfer from the conduction band of Co3O4 to that of CCH, which is verified with a double increase of the photocurrent valve of the heterojunction sample electrode in comparison with the bare Co3O4 sample electrode. Electron paramagnetic resonance, fluorescence spectrophotometry and scavenger experiments indicate that photo-induced holes, and hydroxyl and superoxide anion radicals are the active species responsible for the photo-oxidation of MO. The reasons for the formation of these species are discussed and proposed based on the band gap structures of Co3O4 and CCH. The recycling experiment results indicate that the activity can be regained by a remedial experiment.

 Please wait while we load your content...
Please wait while we load your content...