Reactivity of aminophosphonic acids. Oxidative dephosphonylation of 1-aminoalkylphosphonic acids by aqueous halogens†‡
Abstract
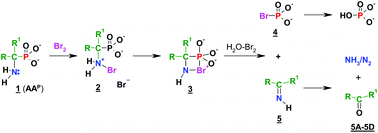
The reactions of 1-aminoalkylphosphonic acids with bromine–water, chlorine–water and iodine–water were investigated. The formation of phosphoric(V) acid, as a result of a halogen-promoted cleavage of the Cα–P bond, accompanied by nitrogen release, was observed. The dephosphonylation of 1-aminoalkylphosphonic acids was found to occur quantitatively. In the reactions of 1-aminoalkylphosphonic acids with other halogen–water reagents investigated by 31P NMR, scission of the Cα–P bond was also observed, the reaction rates being comparable for bromine and chlorine, but much slower for iodine.
- This article is part of the themed collection: Phosphorus Chemistry: Discoveries and Advances

 Please wait while we load your content...
Please wait while we load your content...