Modification of Sn-Beta zeolite: characterization of acidic/basic properties and catalytic performance in Baeyer–Villiger oxidation†
Abstract
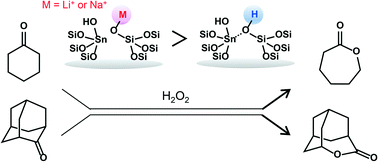
Sn-Beta was post-synthetically modified with various cations such as Li+, Na+, K+, Cs+, and NH4+. During the modification process, the ion-exchange of the cations with silanol groups occurred along with a reversible change between “closed” and “open” Sn sites through hydrolysis of the Si–O–Sn bonds. The IR study showed that Sn-Beta had weak Brønsted acid sites, which were passivated by the ion-exchange, in addition to the Lewis acid sites and that surprisingly, basic sites were formed on the modified zeolites. The ion-exchange with a small amount of Li+, Na+ and NH4+ was effective for suppressing side-reactions, leading to an improvement in selectivity to caprolactone in the Baeyer–Villiger oxidation of cyclohexanone with H2O2. The modification of Sn-Beta with such cations also effectively enhanced the catalytic activity in the Baeyer–Villiger oxidation of 2-adamantanone.
- This article is part of the themed collection: Catalysis on Zeolites

 Please wait while we load your content...
Please wait while we load your content...