Selective oxidation of bulky organic sulphides over layered titanosilicate catalysts†
Abstract
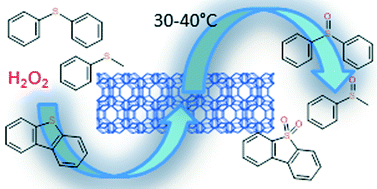
Selective oxidation of sulphides is a straightforward method of preparation of organic sulphoxides and sulphones, which are important chemical intermediates and building blocks of pharmaceuticals and agrochemicals. Oxidation of methylphenyl sulphide (MPS), diphenyl sulphide (Ph2S), and dibenzothiophene (DBTH) over lamellar titanosilicate catalysts with the MFI and UTL-derived topology was investigated with hydrogen peroxide as the oxidant. Lamellar titanosilicates combine the advantages of crystalline zeolites and mesoporous molecular sieves due to accessible active sites located on the external surface of their layers. The selectivity of the MPS oxidation to methylphenyl sulphoxide is driven by the diffusion restrictions in the catalyst. A methylphenyl sulphoxide selectivity of 95% at 40% conversion was achieved using the Ti-IPC-1-PI catalyst together with an outstanding TONtot = 1418 after 30 min. The selectivity can be adjusted also by dosing of the oxidant to keep its concentration low during the reaction. The silica–titania pillared TS-1-PITi catalyst showed the highest potential of the tested catalysts in oxidative desulphuration, easily oxidising the DBTH to dibenzothiothene sulphone.
- This article is part of the themed collection: Catalysis on Zeolites

 Please wait while we load your content...
Please wait while we load your content...